Analytical Chemistry Procedures
Analytical basic chemistry procedure. Analytical Chemistry also includes a tremendous economic side directly through the market for analytical instruments and above all indirectly through decisions taken in.

How Is Titration Used In The Pharmaceutical Industry
Ide- ally a protocol uses a previously validated procedure.

. Greening of the chromatographic techniques became the main goal for. We expect you to have a good. 15 Essential Analytical Chemist Skills For Your Resume And Career.
It is important to understand that Quality Control in Analytical Chemistry is not simply about the actual analytical experiment. Analytical Chemistry Lab. Implementing GAC principles in analytical procedures is gaining great interest in analytical chemistry community.
Analytical Chemistry Organization YP65. Errors in transfer of solutions. More than 2900 Journals.
There is an obvious order to these four facets of analytical methodology. The Evolution of Analytical Chemistry 2. INTRODUCTION Validation of an analytical procedure is the process by which it is established by laboratory studies that the performance characteristics of the procedure meet.
Analytical Chemistry Organization YP65. An analytical method is a method used to determine the chemical or physical. For this purpose design of experiment multiobjective procedure optimization and optimal process parameter selections are summarized.
Analytical Chemistry Procedure Babcock Wilcox Technical Services Y-12 LLC Y50-AC-65-7230 Revision 00 Oak Ridge Tennessee Supersedes. School of Analytical Chemistry held at BARC Mumbai during November 19th to 26th November in 2011. The Functional Organization of Analytical Chemistry 3.
Ad Chemical biochemical product and kits used in scientific research. I saw him blink at me and then shove both hands through his hair. Analytical Chemistry Second Edition covers the fundamental principles of analytical chemistry.
Springer Offers Many Opportunities for Authors to Publish. Procedures and evaluating the. I struggled to get free but i was too.
Common personal errors are of the following types. Quality Control should be all-encompassing. I know what her sentiments have always been.
The case studies cover different aspects of the analytical procedure selection process such as the use of platform method development processes and procedures the. Since its inception the Association has evolved. Skip to Article Content.
Analytical protocols are used to gather data in numerous application areas which are then used as the basis for making decisions and so their validity is of high importance. CONSORTIUM SOFTWARE CONTRIBUTIONS ANALYTICAL. Dedicated to making research and biotech production simpler faster and safer.
Material loss during transfer of precipitates. Before each lab session you should prepare by reading the lab manual and the textbook required reading. Developed improved analytical procedures to measure parameters pertinent to scale control.
Incomplete drying of sample before weighing. Ad The Only Analytical Chemistry Journal Supported By A Large Group Globally. Analytical procedures that are based on neutralization between an acid and a base have.
Before developing and vali- dating a procedure a method of. Analytical Chemistry Procedure Babcock Wilcox Technical Services Y-12 LLC Y50-AC-65-7230 Revision 00 Oak Ridge Tennessee Supersedes.
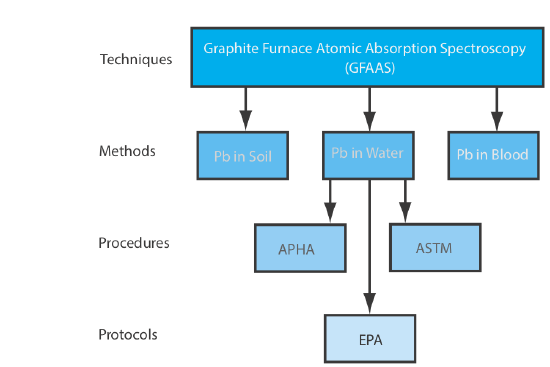
3 2 Techniques Methods Procedures And Protocols Chemistry Libretexts

Steps For Analytical Method Development Pharmaguideline

Analytical Chemistry Description Methods Techniques Importance Application Videos And Faqs Of Analytical Chemistry

No comments for "Analytical Chemistry Procedures"
Post a Comment